Your Donations at Work:
Our Partnership:
Starting in the year 2020, the Castaways have partnered with Sylvester Comprehensive Cancer Center of the University of Miami Health System. Commonly known as “Sylvester,” at time of our partnership it was one of only two NCI-designated* centers in Florida (and 1 of 71 nationwide). In 2022, the Castaways and Sylvester pledged a 5-year partnership to raise $1.5 million dollars which will be directed to funding cancer research.
Sylvester will be matching every dollar we raise (!!) tremendously boosting our impact, while keeping our money local, contributing to research, all with minimal operating costs. Additionally, they will be providing us with reports to see how our money is being put into action which we will be sharing with you, our supporters.
*An NCI-designated cancer designation speaks to the success they’ve had in cancer research and gives them access to cutting-edge clinical trials available exclusively at NCI-designated cancer centers.
Projects Currently Funding:
Targeting Oncogenic Pathways in Chemotherapy-Resistant Sarcoma Cells
PI: Jonathan C Trent, M.D., Ph.D., University of Miami’s Sylvester Comprehensive Cancer Center
$100,000 donation will be made in October 2025.
Original Abstract:
To elucidate the mechanism of drug resistance in human cancer, we will conduct experiments that encompass in vitro 2D- and 3D-culture models as well as generate preclinical animal models for tumor cell metastasis and therapy. Execution of this project over a two-year period will necessitate allocation of funds for analysis and imaging of sarcoma cells and tumor samples, and their implantation in immunodeficient animals for growth and treatment. We will study the signaling pathways responsible for cancer cell resistance to therapy using high-throughput technologies for gene expression and genomic binding to identify and validate novel targets for metastasis therapy. We will establish 2D- and 3D-culture systems that recapitulate the microenvironment of the metastatic sarcoma cell. We will manipulate the identified targets with activators and inhibitors to analyze their effects on cancer cell growth, proliferation, death, invasion, and metastasis within the 2D- and 3D-culture systems. The results of these in vitro assays will then be validated for clinical significance using animal models.
Novel Combinatorial Strategies to Enhance Viral Oncolysis in Renal and Colon Cancers
PI: Jaime Merchan, M.D., University of Miami’s Sylvester Comprehensive Cancer Center
$100,000 donation will be made in October 2025.
Original Abstract:
The goals of this project are to discover novel virus-drug combinations to improve cure rates in advanced cancer. TO achieve this, we will conduct in vitro and in vivo studies using several oncolytic viral platforms and conduct virus-drug screenings, to identify potent combinations that will augment viral replication, cytotoxicity, and immunomodulation. We will use immunocompetent models of kidney and colon cancer, which are two malignancies that are fatal when advanced, and in spite of available treatments, most patients progress and die of refractory disease. Our studies will involve both efficacy and mechanistic analysis of the combinations, with the ultimate goal to translate the lab discoveries into impactful treatment options for patients in our community and beyond.
Evaluating Circulating Tumor DNA for Molecular Response in a Phase II Lymphoma Trial
PI: Juan Alderuccio, M.D., University of Miami’s Sylvester Comprehensive Cancer Center
$100,000 donation will be made in October 2025.
Original Abstract:
As the Associate Professor of Medicine and Clinical Site Disease Group Leader in the Lymphoma Section at the Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Dr. Alderuccio’s institutional obligations include the care of patients with lymphoma, conducting clinical and translational research, and mentoring junior faculty. His translational research focuses on developing decision-making models with imaging, molecular, and clinical data, integrating machine learning approaches. He also conducts clinical trials of novel therapeutics or therapeutic combinations to improve outcomes in patients with difficult-to-treat lymphomas. Consequently, he is an author of more than 65 peer-reviewed manuscripts and has communicated his studies in national and international lymphoma meetings. He is also a member of the American Society of Hematology, where he serves on the Media Experts and Educational Affairs Committees. The funding provided by Castaways Against Cancer will be used to assess the role of a circulatory biomarker called circulating tumor DNA to assess molecular response in a multicenter phase II clinical trial in relapsed/refractory large B-cell lymphoma.
Epigenetics: Pediatric Cancer - Ewing Sarcoma
PI: David Benner Lombard, M.D., Ph.D., University of Miami’s Sylvester Comprehensive Cancer Center
$150,000 donation was made in October 2024.
May 2025 Update:
Our work - generously supported by Castaways against Cancer - focuses on a childhood cancer, Ewing sarcoma. This tumor arises in or around bone, and is always fatal unless treated. Unfortunately, current treatments for Ewing sarcoma have severe long-term side in survivors. Moreover, patients with recurrent or metastatic disease still have a poor prognosis, despite the best current treatments. Most cases of Ewing sarcoma have a specific chromosomal rearrangement, resulting in expression of an abnormal protein, EWS-FLI1. Our studies focus on a cellular enzyme, SIRT5, which we have found stabilizes EWS-FLI1. When we remove SIRT5 from Ewing sarcoma cells, EWS-FLI1 levels drop dramatically, and the cells die. We are developing small molecule inhibitors of SIRT5 as new potential treatments for Ewing sarcoma. Our goal is to optimize these molecules, to serve as new, more effective and less toxic treatments for this cancer.
Original Abstract:
A major focus of our lab is Ewing sarcoma (EWS). EWS is a cancer arising in bone and soft tissue of children and young adults, which is always fatal if not treated. Currently, EWS is treated with a combination of chemotherapy, radiation, and surgery, which can even include limp amputation for some patients. EWS treatments have severe long-term side effects for survivors: accelerated coronary artery disease, heart damage, cognitive problems, and even second cancers. Worse still, patients with metastatic or recurrent EWS have a very poor prognosis, and most will die of their disease. Therefore, there is an urgent need to develop effective new EWS therapies, to cure patients with metastatic cancer, and to avoid the negative consequences of current treatments.
EWS cells possess a unique protein called EWS-FLI1, which is made from a gene fusion that occurs in EWS. EWS-FLI1 functions by binding DNA and turning on expression of genes important for the cancerous properties of EWS cells. EWS cells absolutely depend on EWS-FLI1 for their survival and growth -- if EWS-FLI1 is lost, EWS cells die. Unfortunately, despite intensive research, no means of directly blocking EWS-FLI1 itself has as yet been identified. EWS-FLI1 is a very appealing target for EWS treatment, since normal cells do not possess any EWS-FLI1. Therefore, therapies directed against EWS-FLI1 would likely be very effective at killing EWS cancers, but exert few negative side effects in normal cells and tissues.
Our group studies a protein called SIRT5, which removes a chemical modification called succinylation from its target proteins. Remarkably, we find that SIRT5 regulates succinylation on EWS-FLI1 itself in EWS cells. This effect of SIRT5 is critical for the function of EWS-FLI1. When we inhibit SIRT5, many genes that are normally turned on by EWS-FLI1 are turned off. Crucially, EWS cells lacking SIRT5 rapidly die. Further discovering the details of this SIRT5/EWS-FLI1 link is the focus of our efforts. We think this work is very promising, since it may provide the first practical means to target EWS-FLI1 itself to treat EWS.
To start to bring our basic science findings to the clinic, working with chemists, we have developed new small molecules that block SIRT5 function. When we administer one of these SIRT5 inhibitors to mice with EWS tumors, the cancers stop growing and melt away, while the mice remain healthy. We have recently determined exactly how these inhibitors attach to SIRT5, and based on this knowledge, we are making new, better versions that bind more tightly to SIRT5, in the hopes of making even more effective anti-EWS therapies. We are also beginning to explore roles for other SIRT5-like proteins as targets to kill EWS cells.
Crucially, normal cells and whole mice lacking SIRT5 are healthy. Our work may provide an approach to kill EWS cancers, with little impact on normal tissues, by inhibiting SIRT5 and thereby blocking EWS-FLI1 function. Our long-term goal is to develop new effective treatments for EWS, to spare children with this cancer from the devastating side effects of current treatments, and to save those with recurrent or metastatic EWS, who now too-often succumb to their disease.
Epigenetics: Targeting BRAF & EGFR Mutations in Cancer: Novel Therapeutic Approaches & Challenges
PI: Ramin Shiekhattar, Ph.D., University of Miami’s Sylvester Comprehensive Cancer Center
$150,000 donation was made in October 2024.
May 2025 Update:
Epigenetic Basis of Drug Tolerance - The Mitogen-activated protein kinase (MAPK) pathway is the most frequently mutated signaling cascade in cancer. Inhibitors of oncogenic BRAF, which occurs in ~50% of melanoma patients in combination with MEK inhibitors (Debrafenib/Trametinib; DT), have led to unprecedented clinical responses in patients with BRAF-mutant metastatic melanoma. However, the clinical benefit of such agents is limited by genetic mechanisms that lead to intrinsic or acquired resistance. Additionally, in about 40% of melanoma, cancer cells can escape the effects of targeted therapy through nongenetic adaptive mechanisms. These clinical observations highlight the need for improved understanding of the mechanisms that leads to this cellular reprogramming.
Importantly, we find that genetic or nongenetic resistance in melanoma invariably originates from a pool of transient drug-tolerant cell lineage of staved-like melanoma cells (SMCs). SMCs emerge through phenotype switching following exposure to MAPK inhibitors and, constitutes a cellular pool from which drug resistance ultimately develops. We will dissect the transition of transcriptional and epigenetic landscape of melanoma cells into a drug tolerant SMC lineage during MAPK therapy to gain a mechanistic insight into initiation and maintenance of drug resistance. We will test the hypothesis that therapeutic strategies to target the mechanisms underlying the transition into, and/or maintenance of, the SMC lineage will prevent drug tolerance and, thereby, inhibit the acquisition of resistance in a large fraction of melanoma.
Original Abstract:
Activating mutations in, BRAF and EGFR are the underlying cause of many deadly human cancers. A missense mutation in these genes causes cancers of diverse origins including a large number of lung and skin cancers. The general signaling pathway affected by these mutations, the mitogen-activated protein kinase pathway (MAPK signaling), is a major target of drug design by pharmaceutical companies. However, this approach has been hindered due to the rapid emergence of drug resistance in nearly all cases. To tackle this important human disease there is a need for new groundbreaking unconventional approach to cancer treatment. We propose to establish a new modality for treating these cancers, which we refer to as "Initiation of Transcription Interference or INTi". We believe this plan will be applicable to a wide spectrum of human diseases.
Our proposal centers on our discovery of the functional importance of critical checkpoints during transcriptional initiation mechanisms regulating tissue and disease-specific transcriptional start sites. Aberrant regulation of transcriptional initiation is a key event in the genesis and progression of cancer. We aim to target disease-induced oncogenes to silence cancer-causing genes as a treatment in cancers induced by activating mutations in cancers displaying resistance to targeted therapy. We will develop derivatized anti-sense oligonucleotides (ASOs) that will neutralize the oncogenic transcripts in vivo allowing for disease-specific inhibition of pathogenic gene expression in cancer.
Epigenetics: Chromosomal Regulation in Leukemia & Breast Cancer
PI: Martin A. Rivas, Ph.D., University of Miami’s Sylvester Comprehensive Cancer Center
$150,000 donation was made in October 2023.
May 2025 Update:
Metastatic dissemination–the spreading of cells outside of a primary tumor to seed a distal organ–is a grim milestone in breast cancer progression: in fact, metastatic breast cancer remains incurable, underscoring the need for additional research aiming at preventing cells from establishing distal metastasis. In our lab, we are interested in understanding how genome folding, i.e., how the almost two meters of DNA contained in every cell is packaged, influences cell characteristics, including malignant traits such as dissemination. We think that the key to preventing primary tumor cells from becoming metastatic lies in high-order structural changes that occur in the chromosomes.
Over the last two years, we have made significant progress in understanding how chromosomal architecture makes epithelial breast cancer cells more migratory and contributes to metastatic dissemination. Using an in vitro system, we characterized the changes in genome folding that occur when cells gain metastatic traits. We have also evaluated how genome folding is affected when the molecular machinery (the cohesin complex) that folds the DNA is purposefully deactivated, producing the first ever genome-wide maps of contact in identical cells that only miss a cohesin subunit. We have seen that our results are reproducible over several models, and we are currently performing in vivo studies.The support from the Castaways Against Cancer has been crucial to kickstart this project, and we are deeply grateful for your support at such a critical stage.
July 2024 Update:
*Click here for PDF version of this update.
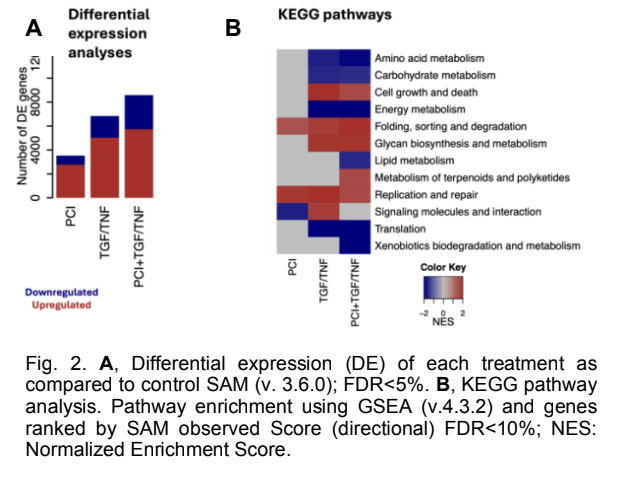
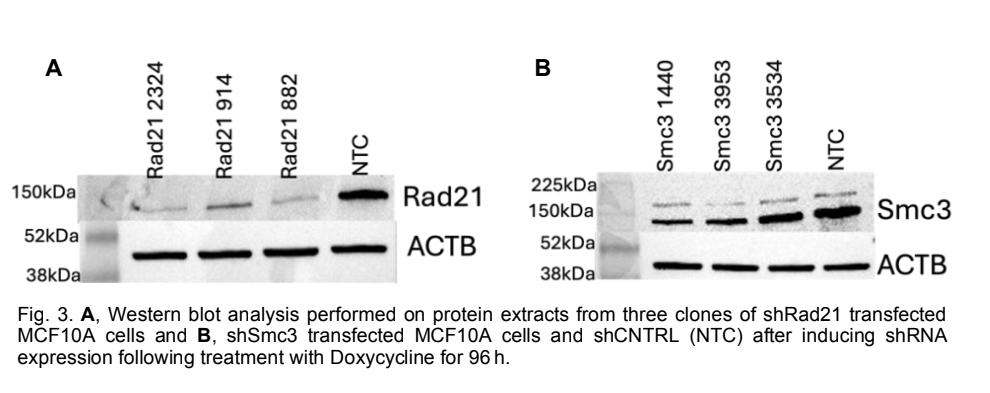
Over the past year, we have made progress on several projects related to the mechanisms by which chromosomal architecture regulates physiological and pathological mechanisms during cancer initiation and progression. In particular, we have explored the role of cohesin and chromosomal architecture during the metastatic dissemination of breast cancer. We established the EMT protocol in MCF10A cells by treating the cells with TGF- /TNF- for 6 days. The transition was confirmed by a striking morphology difference with and without the treatment and Western blot, as shown in Fig. 1A. Western blot showed downregulation of epithelial marker E-cadherin and increased expression of mesenchymal markers Fibronectin, Vimentin, and transcription factor Snail following the treatment (not shown). To investigate the involvement of cohesin in EMT firsthand, PCI34051 was introduced in the presence and absence of EMT induction. PCI34051 is a drug that inhibits HDAC8, a protein that deacetylates Smc3, a subunit of the cohesin complex, along with SMC1A, RAD21, and STAG1/STAG2. By inhibiting HDAC8, the cohesin loading and unloading cycle is disrupted. We observed a partial reduction in the expression of Fibronectin by Western blot (Fig. 1B). However, other proteins, such as Vimentin and E-cadherin levels, remained unchanged by the presence of PCI- 34051. This shows the possible involvement of the cohesin complex in the progression of EMT and raises the question of the exact mechanism by which this occurs. In addition, we produced RNA-sequencing, global Hi-C, and promoter-capture Hi-C to interrogate the changes in genome architecture and gene expression during EMT. Principal component analysis revealed distinct clustering of RNA-seq samples based on treatment, suggesting robust transcriptional differences. There was a 14% clustering difference between the control and the PCI treatment, whereas that of the TGF-/TNF- and the combined treatment was 50%. There were 11,735 differentially expressed genes between the three treatment conditions at an FDR<5%. As shown in Fig. 2A, with TGF-/TNF-, about 5000 genes were upregulated and 2,000 downregulated, and a slightly higher number was observed for the combinatorial treatment of TGF- /TNF- + PCI. Pathway analysis identified key signaling and metabolic pathways such as cell growth and death, lipid metabolism, amino acid metabolism, and translation pathways, with the treatment conditions displayed in Figure 3B. We have also stably transfected MCF10A and MDA-MB-231 cells with short hairpin RNAs against Rad21, Smc3, and a nontargeting control. We induced the expression of the shRNAs gradually by treating the cells with doxycycline for 96 h. To ensure the stable knockdown of the proteins, we viewed the cells under a fluorescent microscope and ran a Western blot for three clones of Rad21, Smc3, and the control (NTC).
Original Abstract:
Regulation of transcriptional programs and gene expression is tightly controlled at the epigenetic level. Chromosomal architecture has arisen as a hierarchical epigenetic regulator by physically segregating transcriptional domains into active or inactive compartments. Such regulation is performed by the cohesin complex and by the transcription factor CTCF. The research program of my laboratory focuses on how chromosomal architecture regulates physiological and pathological mechanisms during cancer initiation and progression. The overarching goal of my research is to elucidate the chromosomal architecture mechanisms driving malignant transformation, as a way to identify actionable therapeutic targets in cancers with alterations of the nuclear topology. Some projects related to this overarching goal will explore:
i) Oncogenic role of PDS5B and CTCF mutations in B-cell development and lymphoma. Ongoing work explores how mutations in cohesin unloader PDS5B and CTCF can affect humoral immunity and impair immunoglobulin-producing plasma cell differentiation. We are also assessing the tumor suppressor function of CTCF haploinsufficiency and the oncogenic potential of R289H mutation of PDS5B in combination with lymphoma oncogene.
ii) Functional role of cohesin mutations in pediatric acute megakaryoblastic leukemia (AMKL). Mutations in cohesin are a hallmark of Down Syndrome-related AMKL, occurring in the transformed phase of the disease but not in the transient abnormal myelopoiesis. However, functional implications of cohesin mutations in AMKL transformation has not been systematically explored. This project uses animal models and genome editing to produce isogenic systems to address these questions.
iii) Role of cohesin and chromosomal architecture during the metastatic dissemination of breast cancer. Metastatic dissemination involves plastic changes in cell phenotype, in particular, during the epithelial-to-mesenchymal transition that enables cell seeding and colonization of secondary sites. This project explores the involvement of cohesin and 3D genome topology during the process of EMT. Given the universality of these molecular mechanisms, we anticipate that these findings could be extensible to other cancer types, increasing the translational potential and impact of our research.
Epigenetics: Blood Cancer
PI: Luisa Cimmino, Ph.D., University of Miami’s Sylvester Comprehensive Cancer Center
$150,000 donation was made in October 2023.
$100,000 donation was made in October 2022.
May 2025 Update:
With generous support provided by the Castaways Against Cancer the Cimmino lab has prepared three research studies for publication including 1) Elevated B12 serum levels are associated with the presence of Clonal Hematopoiesis by Gupta et al, under review at the journal Blood Neoplasia 2) One-carbon metabolism promotes gut dysbiosis and an inflammatory microenvironment that potentiates Tet2-deficient hematopoiesis by Lyon et al, that was reviewed at Nature Communications and 3) Retinoic acid and ascorbate synergize to suppress myeloid leukemia via TET2 activation by Leesang et al. that was reviewed at Blood Cancer Discovery. Dr. Cimmino is the senior and corresponding author on these reports. These publications are a culmination of research in the Cimmino lab funded by the Castaways Against Cancer focused on how metabolism and micronutrients influence blood cancer progression to identify targeted interventions for clinical translation. Dr. Cimmino has been recruited as a Guest Editor for the research journal “Nutrients” to develop a Special Issue on “The Role of Nutritional Supplements in Cancer Prevention and Treatment”. She was also invited to present her work at an NIH symposium on the use of “Pharmacological Ascorbate and Cancer” in November 2024 and at the annual American Society for Hematology Meeting held in San Diego in 2024 to present her work on “Targeting TET2 for the treatment of Clonal Hematopoiesis” in a workshop on Clinical Trial Approaches in Clonal Hematopoiesis. She is currently preparing to translate her work into a multi-site clinical trial at the Sylvester Comprehensive Cancer Center in collaboration with Moffit and The Mayo clinic to treat clonal cytopenia.
July 2024 Update:
Support from the Castaways Against Cancer has allowed the Cimmino lab to expand their research on how metabolism and micronutrients influence blood cancer progression to identify targeted interventions for clinical translation. Work form the lab was presented at the International Society of Experimental Hematology and American Society of Hematology annual meetings in 2023 (https://doi.org/10.1182/blood-2023-189774), and the lab has recently published a review summarizing how dietary nutrients can influence the epigenome (https://doi.org/10.3389/freae.2024.1409355). The Cimmino lab has recruited additional PhD and Post-doctoral trainees to work on NIH-funded research that was obtained thanks to Castaways seed funding. Dr Cimmino was also awarded Mentor of the Year for Trainees in 2023 by the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine.
August 2023 Update:
Vitamins play an essential role in keeping our immune system healthy by maintaining normal blood cell production. Certain types of vitamins can also help in the prevention and treatment of blood cancers. Vitamin A in the form of retinoic acid has been used for decades to treat a subset of blood cancer patients with defects in a protein that relies on vitamin A for its normal activity. More recently, our work has shown that vitamin C can also stop blood cancers from forming, and slow cancer growth, by maintaining or restoring the activity of a protein known as TET2. Loss of TET2 function causes an increase in the growth of blood cells that drive cancer development. Mutations in TET2 that lower its activity are frequently found in patients with blood cancers. TET2 is also frequently defective in the blood cells of the healthy elderly population that can put them at a much greater risk of developing a blood cancer. The goal of our work is to understand how we can maintain TET2 activity to prevent and block cancers of the blood. Vitamin A treatment has recently been shown to increase TET2 levels in cells, which in combination with vitamin C restores TET2 activity more than either treatment alone to stop the growth of blood cancer. Our goal in this study is to model combination treatment strategies of vitamin A and vitamin C to prevent blood cancer formation and growth that can be used as a potential therapy to treat blood cancer patients with a loss in TET2 activity.
May 2023 Update:
Research funded by the Castaways in the Cimmino Lab has contributed to an NIH National Cancer Institute funded 5 year grant of $1.25 million to determine how to enhance the activity of a tumor suppressor, TET2, for the prevention and treatment of blood cancers.
The success of this project in gaining federal funding allows us to expand upon this research, which could only have been possible thanks to the generous donation from the Castaways.
Orginal Absract:
My research is focused on understanding how epigenetic dysregulation, such as aberrant DNA methylation, drives blood cancer progression, and whether these epigenetic alterations impart vulnerabilities that can be targeted for therapeutic intervention. Dietary nutrients such as vitamins and amino acids influence blood cell production by participating as acceptors and donors of one-carbon metabolism, and as cofactors of epigenetic enzymes that write and erase DNA methylation in the mammalian genome. Using cellular and animal blood cancer models, coupled with high-throughput genomic and chemical screening approaches, our studies have revealed several combinatorial treatment strategies that can be implemented to enhance existing therapies for the treatment of myeloid leukemia. We also show that aberrant DNA methylation in blood cancer cells can be targeted therapeutically through dietary intervention by altering micronutrient supplementation. These studies reveal potentially novel strategies to therapeutically target epigenetic dysregulation for the prevention and treatment of blood cancers.
Epigenetics: Breast Cancer
PI: Lluis Morey, Ph.D., University of Miami’s Sylvester Comprehensive Cancer Center
$100,000 donation was made in October 2022.
July 2024 Update:
The funding from Castaways Against Cancer has been critical in advancing the research of the Morey lab on developing new therapies for breast cancer. Last year, we published a paper in Nature Structural & Molecular Biology that paves the way for the development of a novel therapy to treat metastatic breast cancer. More recently, thanks to Castaways Against Cancer, we have been able to focus our efforts on developing new tools and discovering new genes that are key to ensuring the efficacy of immunotherapy and chemotherapy for patients with the most aggressive type of breast cancer, triple-negative breast cancer.
May 2023 Update:
Our lab investigates the epigenetic dependencies and vulnerabilities in endocrine-sensitive and endocrine-resistant breast cancer, with the goal of identifying novel therapeutic targets and mechanisms to improve the overall survival of patients with breast cancer.
Original Abstract:
The overarching goal of our lab is to identify effective therapeutic strategies to improve the overall survival of patients with metastatic breast cancer, a disease that will claim the lives of 500,000 women across the world every year. The incidence of invasive breast cancer has been increasing since 2004, with more than 2 million cases reported worldwide in 2022. More than 290,000 U.S. cases will be diagnosed in 2022. Fortunately, due to improvements in screening, diagnosis, and treatment, the mortality rate of invasive breast cancer is decreasing in recent years. Treatment differs between the molecular subtypes of breast cancer which are defined by gene expression and clinically approximated by the evaluation of biological markers, primarily the hormone receptors ER (estrogen receptor) and PR (progesterone receptor), as well as the epidermal growth factor receptor HER2. Based on expression of these receptors, breast cancer can be classified into five major molecular subtypes: luminal A, luminal B, HER2-enriched (HER2e), basal-like, and normal-like. In contrast, basal-like breast cancers do not express ER, PR, or HER2, and are therefore referred to as triple-negative breast cancer (TNBC). In ER+ breast cancer, endocrine therapy has proven successful in the treatment of hormone-responsive breast cancer since its early adoption in the 1940s as an ablative therapy. Despite the efficacy of endocrine therapy, resistance arises in about 30% of patients with early-stage disease and in almost all patients who develop metastasis, leading to poor clinical outcome. Overcoming these outcomes is a major challenge in the ER+ breast cancer therapeutic arena. In our efforts to identify new potential therapeutic targets, we discovered that the epigenetic complexes CoREST and Polycomb are key determinant of resistance to endocrine therapies in breast cancer. Notably, genetic, and chemical inhibition of CoREST and PRC1 complexes, inhibit proliferation, primary tumor, and metastasis of endocrine resistant breast cancer and TNBC. We are currently investigating the CoREST and PRC1-mediated molecular mechanisms that lead to oncogenesis in breast cancer. With the support of Castaways Against Cancer, our research program will reveal novel potential therapeutic options for treating metastatic breast cancer patients.
Epigenetics: Blood Cancer associated alterations that occur in the nuclear export protein, XPO1
PI: Justin Taylor, M.D., University of Miami’s Sylvester Comprehensive Cancer Center
$100,000 donation was made in October 2022.
July 2023 Update:
The 2023-2024 year was a big year for the Taylor lab with funding from Castaways Against Cancer playing into their success. Dr. Taylor received a Maximizing Investigators’ Research Award (MIRA) from the National Institutes of Health (NIH) for his research on “The role of XPO1 in nuclear export of RNA” (https://news.med.miami.edu/nih-award-to-research-how-key-protein-transport-mechanism-goes-awry-in-cancer/). Dr. Taylor also received the Schally Research Award from the University of Miami, named for the Nobel laureate Dr. Andrew Schally. In February of 2024, Dr. Taylor and colleagues published their findings on “Kinase-impaired BTK mutations are susceptible to clinical-stage BTK and IKZF1/3 degrader NX-2127” in Science https://www.science.org/doi/full/10.1126/science.adi5798 https://news.med.miami.edu/study-investigates-leukemia-drugs/ Dr. Skye Montoya, PhD received her doctoral degree from the University of Miami based on her work in Dr. Taylor’s lab and was honored with the top graduate student award from the University of Miami. https://news.med.miami.edu/medical-faculty-association-best-research-awards/ https://news.med.miami.edu/justin-taylor-skye-montoya-mentorship/ With continued support from the Castaways Against Cancer, Dr. Taylor hope that more students and other scientists will advance in their training and go on to continue making new discoveries in the fight against cancer.
May 2023 Update:
The funding from the Castaways Against Cancer has been critical to moving forward the research of the Taylor lab on developing new therapies for blood cancers. Last winter we published a research article “Combination venetoclax and selinexor effective in relapsed refractory multiple myeloma with translocation t (11; 14)” in Nature Precision Oncology. This translational laboratory research led to the development of a clinical trial that is now open at Sylvester Comprehensive Cancer Center (ClinicalTrials.gov Identifier: NCT05530421). We also presented our research on “Kinase Dead BTK Mutations Confer Resistance to Covalent and Noncovalent BTK Inhibitors but Are Susceptible to Clinical Stage BTK Degraders” at the American Society of Hematology Annual meeting in New Orleans, LA. This research is now being submitted for publication and is being tested in clinical trials across the country (ClinicalTrials.gov Identifiers: NCT04830137, NCT05131022). With the continued support of the Castaways Against Cancer we are launching several new research projects that will hopefully translate into new approved therapies for patients with blood cancer. Finally, Dr. Taylor received the Mentor of the Year award from the Department of Medicine at the University of Miami Miller School of Medicine.
Original Abstract:
New therapies for patients with cancer who have relapsed after standard treatment is an urgent unmet need. Alterations of the nuclear export protein XPO1 in cancer include mutations and overexpression and are particularly seen in blood cancers. We have previously observed high expression of XPO1 in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) that is associated with worse outcomes. We are trying to understand one of the mutations that occurs in XPO1, which is important for an essential cellular process called nuclear export, but it is unclear how these mutations lead to cancer. Our preliminary data identify that mutant XPO1 is more sensitive to anti-cancer drugs that inhibit nuclear export by binding XPO1. We have also identified mutations in the splicing factor gene SF3B1 as predicting sensitivity to the clinical use of the FDA-approved XPO1 inhibitor selinexor. We hope that research into the process by which these mutations lead to sensitivity to XPO1 inhibition will help us to discover better ways to provide treatment to patients whose cancer is found to have XPO1 or SF3B1 mutations (which are common in leukemias) or to a broader group of patients by using combination therapies.
Targeted therapies that selectively inhibit XPO1 are approved for use in related blood cancers such as multiple myeloma and lymphoma but have not yet successfully been developed in leukemia and MDS. Furthermore, animal models and detailed mechanism of action studies are lacking, potentially slowing the translation towards new clinical therapies and combination therapies for patients. To address these problems, we will also investigate whether XPO1 inhibition perturbs RNA export and how that affects gene expression, splicing, and translation given the known role of XPO1 to export certain messenger RNA, small nuclear RNA, and ribosomal RNA species via RNA-binding proteins. Lastly, we will use our models to test potential targets for combination with XPO1 inhibition in an unbiased manner. This is directly relevant to advancing progress towards cures for cancer and dramatically improving the mortality of leukemias and myelodysplastic syndromes. Funding from the Castaways Against Cancer will greatly impact our research to speed these discoveries and can be expected to have a direct and significant positive impact on the field of precision medicine in AML and MDS.
Projects Funded in the Past:
Lymphoma research
PI: Jonathan Harry Schatz, MD, University of Miami’s Sylvester Comprehensive Cancer Center
$110,000 donation was made in August 2021.
*Here is an article from the BBC on successful work in the same field as Dr. Schatz’s research. Amazing and heartwarming story demonstrating the research being done here is valuable.
May 2023 Update:
Here is the link to an article announcing how Dr. Schatz received a $1.5 million award from the U.S. Department of Defense Office of Congressionally Directed Medical Research Programs based on his finding on this research project. This provides a great update on his incredible progress.
September 2022 Update:
We are using Castaway funds to support a project to better understand and improve responses by aggressive lymphoma tumors to a new type of immunotherapy called chimeric-antigen receptor (CAR)-T cells. These innovative therapies take immune cells from individual patients and reprogram them to attack their tumors. Reinfusion of these cells will lead to responses in many patients lacking other treatment options, and 30-40 percent will experience long-term remissions. Reasons for failed treatments in other patients are poorly understood and are an intense area of research in the field. We recently published data on the genomes of lymphoma tumors treated with CAR-T cells and found striking findings that associate with responses. Now we must perform laboratory studies to understand how these findings drive treatment resistance.
We therefore are using laboratory model systems to carry out functional studies of specific changes to the genome. We believe specific alterations cause tumors to promote cellular environments in which they are protected from immune cells that otherwise would be able to target and eliminate them. A specific tumor-suppressor gene called RHOA is a major focus of current studies. We have modeled genomic deletion of RHOA that is seen in patients with tumors resistant to CAR-T cells and find specific reprogramming of gene expression by tumors that is hostile to their efficacy. Next, we are moving these studies into mouse models so that we capture better overall pictures of immune function and test efficacy of CAR-T cells in way that can inform the treatment of patients once mechanisms are defined. We already have used data generated with Castaways support to apply for a large grant from the National Cancer Institute and are planning multiple additional applications for external funding. Castaways’ support has been instrumental in getting these highly clinically relevant research studies off the ground.
Original Abstract:
Dr. Schatz’s work focuses on the non-Hodgkin lymphomas, a group of more than 60 cancers arising from B and T lymphocytes. He seeks to identify and understand the molecular mechanisms that cause resistance to specific therapies, causing treatment failures in patients. Current work includes targeted therapies, immunotherapies, and traditional chemotherapies. By understanding resistance at a core molecular level, Dr. Schatz hopes to develop new treatments that overcome these barriers to better outcomes for patients.
Establishing a CHIP clinic for early cancer detection.
PI: Dr. Namrata Sonia Chandhok, MD, University of Miami’s Sylvester Comprehensive Cancer Center
$110,000 donation was made in August 2021.
May 2023 Update:
Greetings! I am writing to express my heartfelt gratitude for the generous funding you provided for my cancer prevention research project. As a reminder, I have been working on developing a program to study clonal hematopoiesis, or CH. CH is a condition where a group of cells in an individual’s bone marrow, responsible for producing blood cells, starts behaving differently than normal. Because of this, a small population of cells can become dominant, and in some cases can lead to development of various blood cancers. Not everyone with these abnormal populations will develop a cancer, and in collaboration with large research groups across the country we are working to identify those who are at the highest risk of progressing to a blood cancer. I am a leukemia specialist, and therefore my primary interest is to better understand which patients are at high risk for develop myelodysplastic syndrome and acute myeloid leukemia. We know that many people who receive chemotherapy for other diseases are also at high risk, and therefore I am working with specialists in different disease groups to help reduce the risk of developing this disorder. Your support has led to an established CH clinic at UM where we can evaluate patients who are at high risk for blood cancers and other disorders, and with their permission use their blood samples to understand changes we can make to reduce their risk of disease. We will also offer clinical trials to reduce these abnormal populations of cells.
The importance of cancer prevention cannot be overstated. By investing in research that focuses on preventing cancer, we have the opportunity to make a profound impact on countless lives. Your support has been invaluable in advancing my efforts to help prevent these devastating diseases that are traumatic for patients and caregivers alike.I am honored to be associated with an organization that not only recognizes the significance of cancer research but actively takes steps to make a difference. Your generosity and compassion serve as an inspiration to continue my work with renewed passion and dedication.
September 2022 Update:
I would like to take this opportunity to thank you again for your generous support for our research efforts. As you may recall, I am a leukemia physician and my research is focused on treatment of these blood diseases with innovative, as well as identifying preventative measures to prevent cancer development. The grant funding provided by your efforts has helped establish a clonal hematopoiesis clinic that is now clinically active and I am currently seeing patients with precancerous mutations. With the support of patients with these mutations, we are analyzing blood samples, and occasionally bone marrow biopsy samples, to identify patterns that may be important to predict cancer risk. In the future, we also hope to provide treatment modalities that lower the risk of cancer development. We have also created collaborations with major cancer centers across the country to accelerate these efforts, and further magnify the results that will stem from your contributions.
Original Abstract:
In addition to developing cutting-edge therapies to improve the lives of patients affected by cancer, our aim is to stop cancer before it starts. Increasing age, certain exposures, and common illnesses can lead to genetic mutations in the stem cells that produce blood. These mutations can lead to the development of abnormal blood cell populations known as “clonal hematopoiesis”. Clonal hematopoiesis has been linked to serious health problems like heart disease and the development of blood cancers like myelodysplastic syndrome and acute myeloid leukemia in some patients.
Your generous contribution to Sylvester Comprehensive Cancer Center will help us establish a clonal hematopoiesis clinic to screen patients at high risk for these abnormal blood cell populations, employ known risk reduction strategies to prevent life-threatening illness in these patients, and develop new cancer prevention strategies.
Computational Approaches for Identifying Effective Treatments for Pediatric Brain Tumors
PI: Zane Zeier, PhD., University of Miami’s Sylvester Comprehensive Cancer Center (Former PI: Nagi Ayad, Ph.D.)
$100,000 donation was made in August 2020.
Dr. Ayad’s update on Jan 15th, 2021 (video below)
November 2021 Update: Click here
Original Abstract:
Medulloblastoma (MB)is the most common pediatric brain tumor. MB standard of care includes surgery, followed by radiation of the brain and spinal cord, and adjuvant chemotherapy. Although survival benefit occurs for some patients after standard-of-care treatment, several deficits persist. Treatment sequalae include neurocognitive impairments, mutism, and hearing loss, as well as secondary malignancies that arise. Importantly, some patients are resistant to conventional therapy. Thus, there is considerable interest in identifying new therapies for treating MB patients. MB has been classified into four major subgroups: WNT, SHH, Group 3 and Group 4, each with its own histology, molecular drivers and prognoses. We have recently developed a computational pipeline to identify therapeutic combinations in a patient specific manner. This pipeline, termed SynergySeq, allows us to stratify patients based on the tumor makeup. We have used this pipeline effectively for glioblastoma, the most common adult brain tumor, to make predictions that were confirmed in preclinical models. We now would like to apply this same computational pipeline to pediatric brain tumors.
Although some children who suffer from MB go on to lead a healthy life after surgery and radiation, some children do not respond to this treatment and succumb to this disease. Therefore, we are trying to find safe and effective therapies for those patients. One of the main issues with brain tumors is that tumors are made up of many different cells and this makes it difficult to ascertain which cells to try to eliminate with a drug. We have developed a way to find this out based on sequencing each cell individually within MB tumors. We will use novel computational approaches we developed to identify FDA approved drugs to target the cells in medulloblastoma and test these drugs in animal models of MB. These preclinical studies will make way for clinical trials in MB.